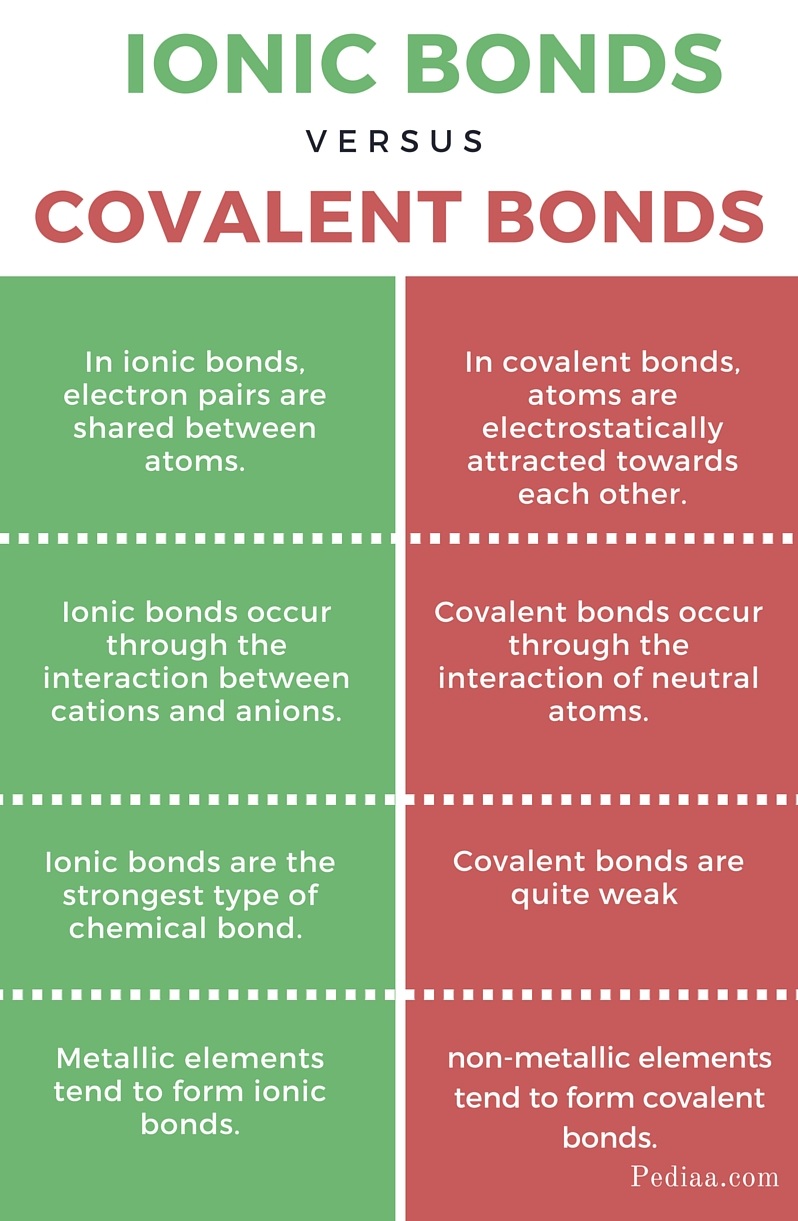
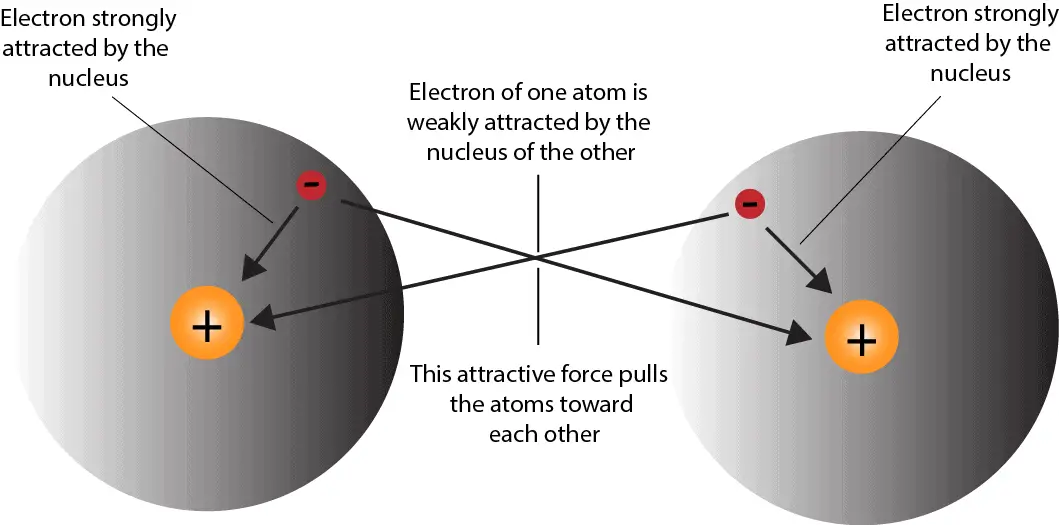
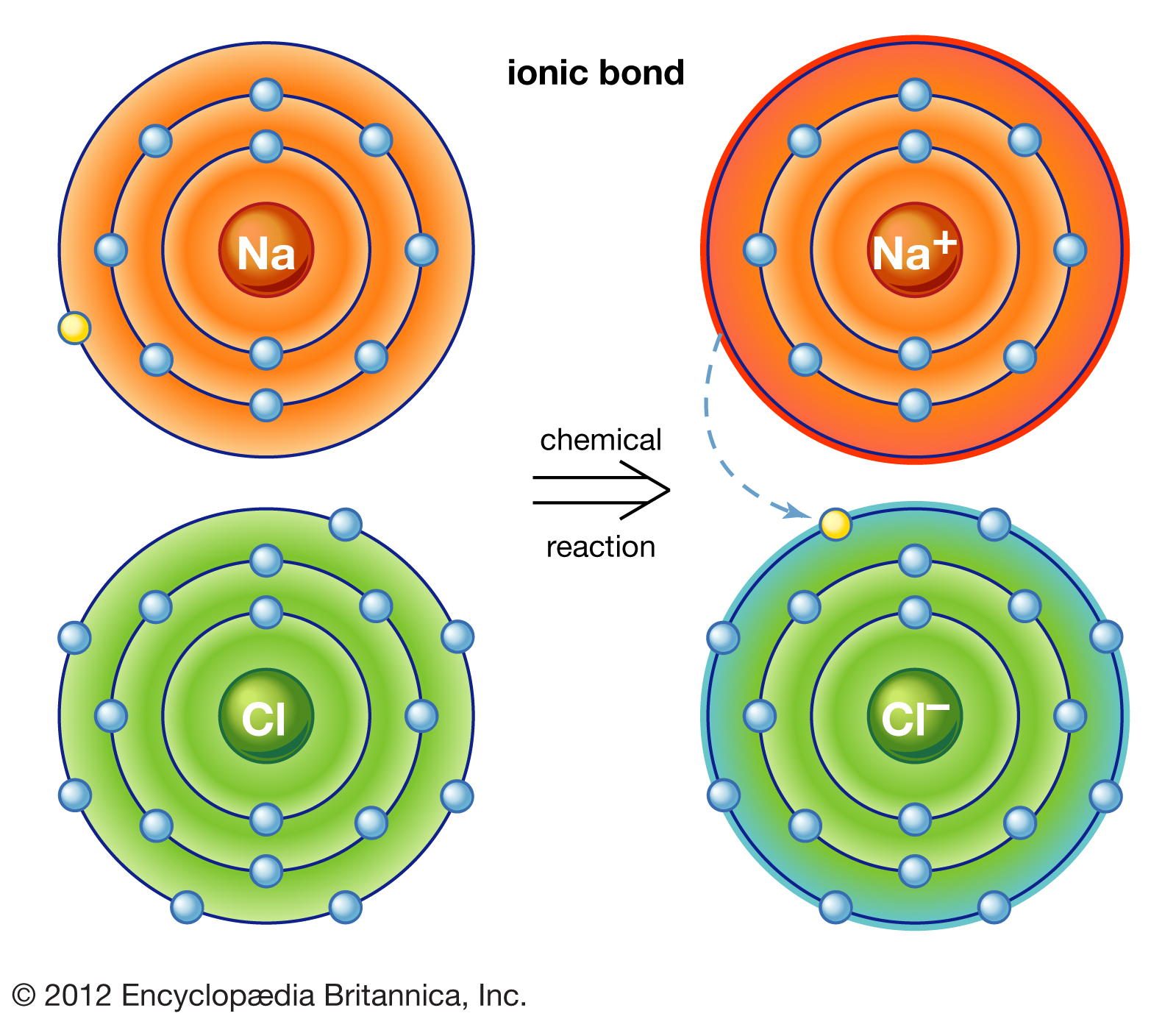
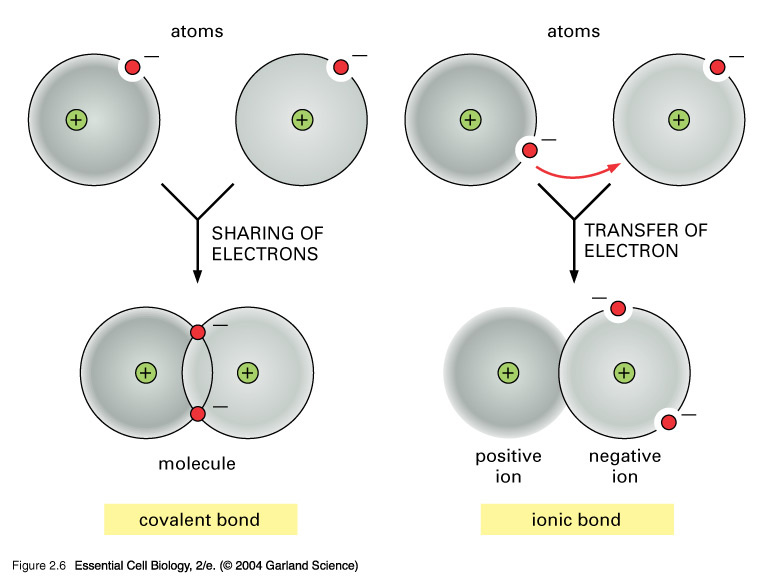
Why Do Atoms Form Ionic And Covalent Bonds - Charged atoms are called ions. In ionic bonding, atoms transfer electrons to each other. Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron.
In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions. Ionic bonds require at least one electron. Because opposite charges attract (while like charges repel), these.
Because opposite charges attract (while like charges repel), these. Charged atoms are called ions. Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other. Because opposite charges attract (while like charges repel), these. Charged atoms are called ions.
Compounds With Both Ionic and Covalent Bonds
Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other. Because opposite charges attract (while like charges repel), these. Charged atoms are called ions.
Difference Between Covalent and Ionic Bonds
Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions.
How do atoms form covalent bond?
In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions. Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron.
Reading Covalent Bonds Biology I
Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions. Ionic bonds require at least one electron.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Charged atoms are called ions. Ionic bonds require at least one electron. Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other.
Covalent Bond Is Between Covalent Bonds vs. Ionic Bonds What is The
Ionic bonds require at least one electron. Charged atoms are called ions. Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other.
chemistry knowledge Comparison between Covalent and Ionic Bond
Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions.
How To Form Ionic Bonds
In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions. Ionic bonds require at least one electron. Because opposite charges attract (while like charges repel), these.
In Ionic Bonding, Atoms Transfer Electrons To Each Other.
Ionic bonds require at least one electron. Because opposite charges attract (while like charges repel), these. Charged atoms are called ions.