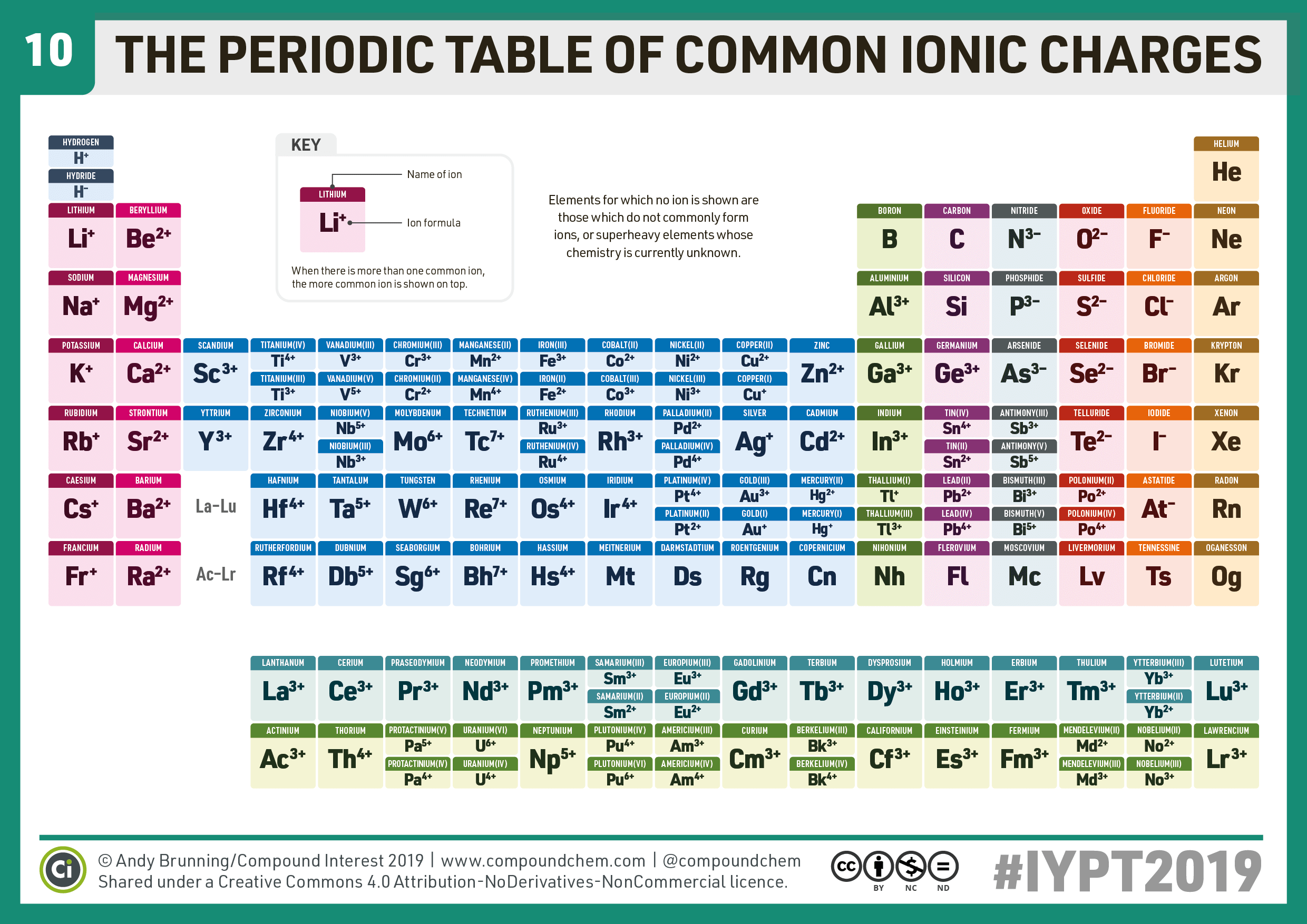
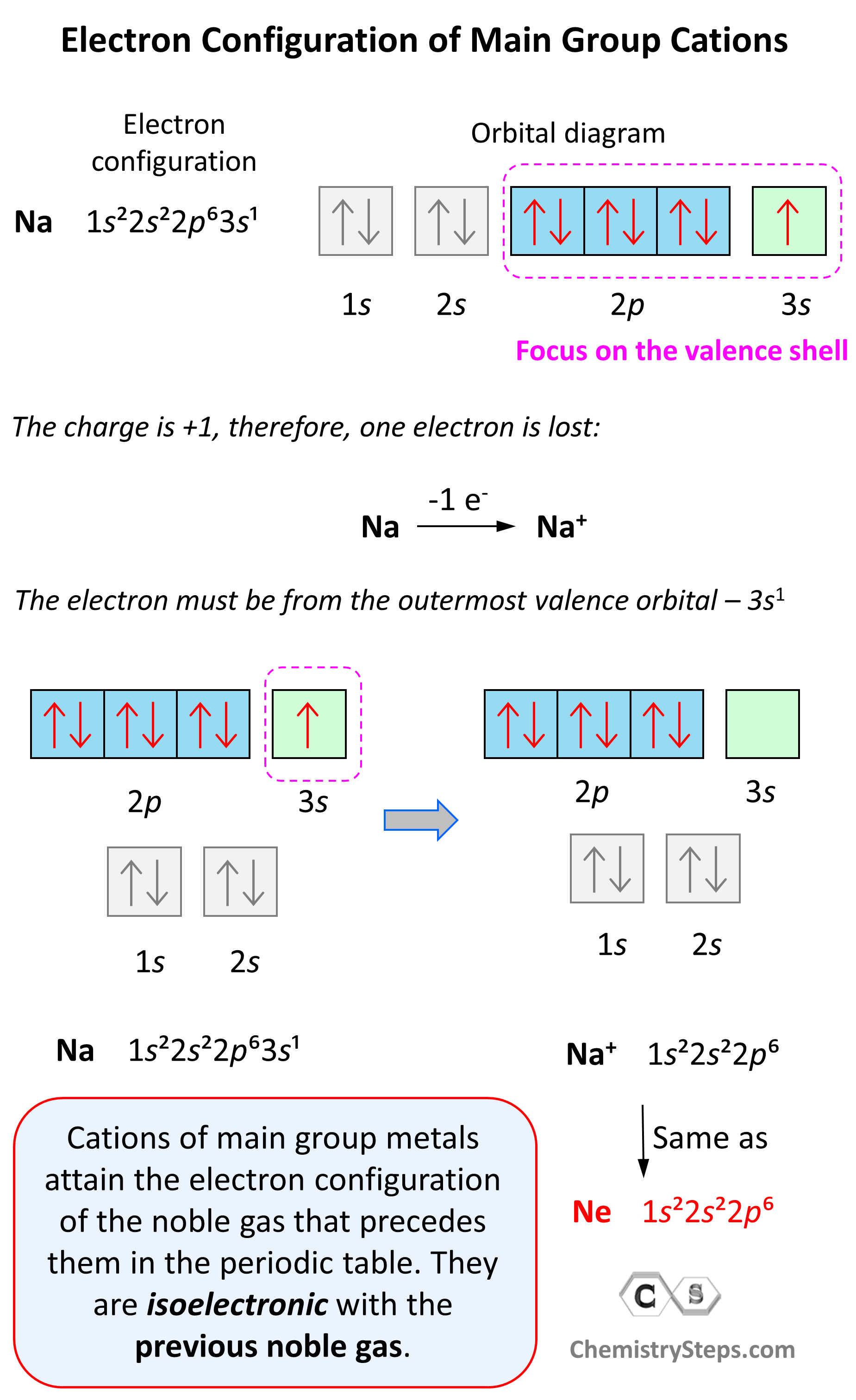
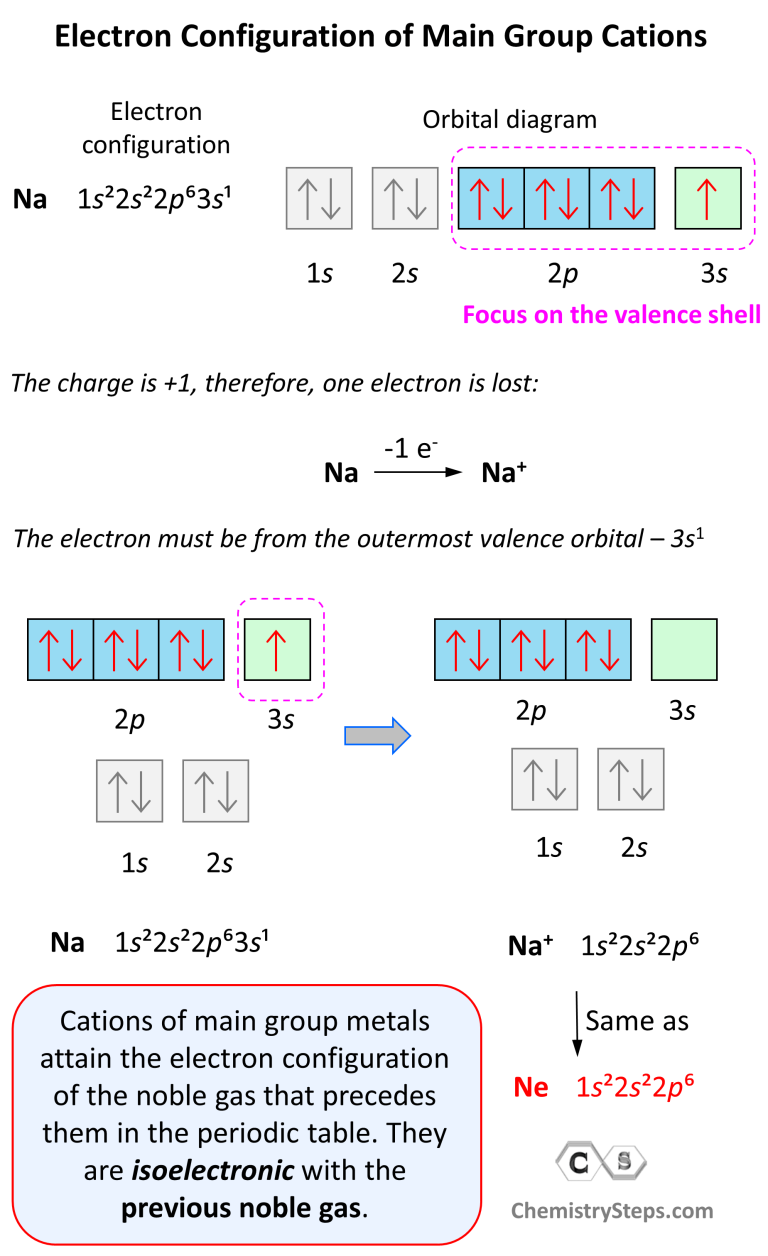
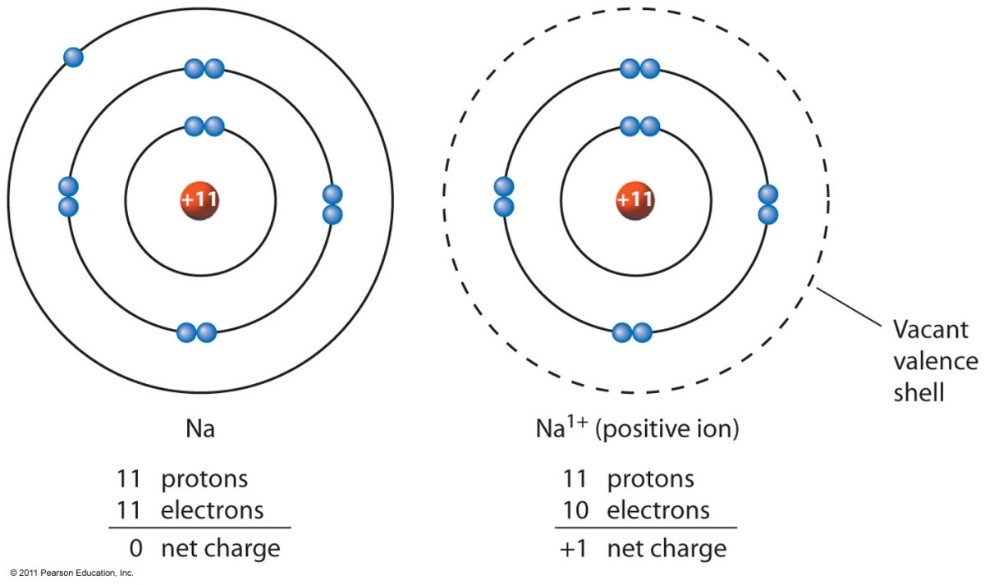
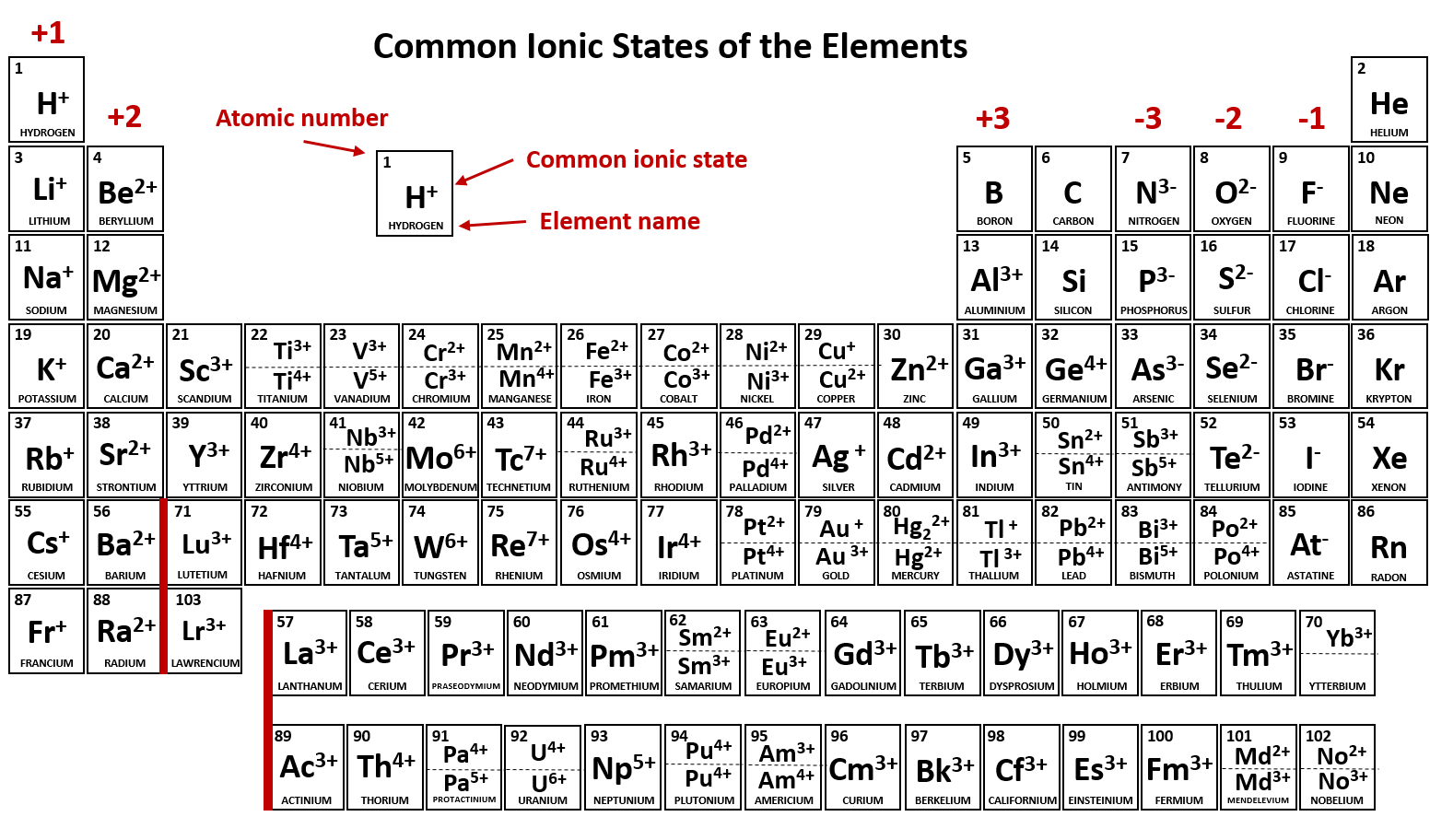
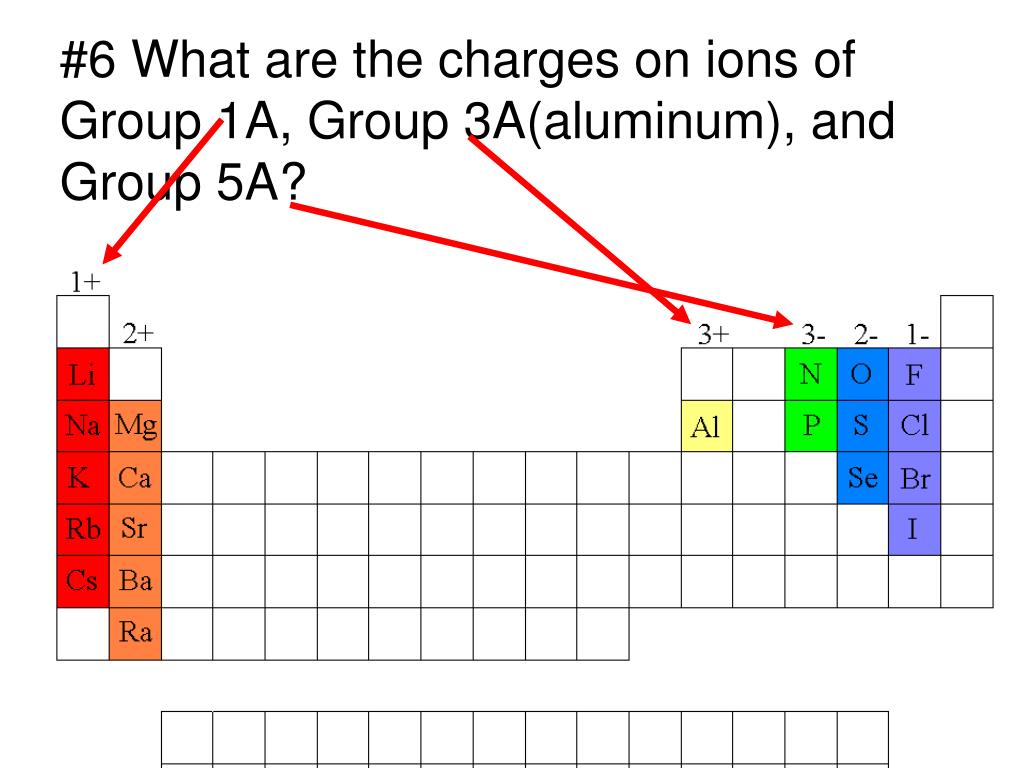
Which Group Tends To Form 1 Ions - Which periodic group forms +1 ; The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which group tends to not form ions or react? Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$.
The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which group tends to not form ions or react? Which periodic group forms +1 ; The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Study with quizlet and memorize flashcards containing terms like which group tends to.
Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Which periodic group forms +1 ; Which group tends to not form ions or react? Study with quizlet and memorize flashcards containing terms like which group tends to. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +.
Naming Simple Ionic Compounds Pathways to Chemistry
Study with quizlet and memorize flashcards containing terms like which group tends to. The charge on a ion indicates the no. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which group tends to not form.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Study with quizlet and memorize flashcards containing terms like.
Compound Interest 10 Periodic Table of Common Ions
Which periodic group forms +1 ; Which group tends to not form ions or react? Study with quizlet and memorize flashcards containing terms like which group tends to. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. The charge on a ion indicates the no.
Chem Ions Scientific Tutor
Which group tends to not form ions or react? Which periodic group forms +1 ; The charge on a ion indicates the no. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Study with quizlet and memorize flashcards containing terms like which group tends to.
Electron Configurations of Ions Chemistry Steps
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Which periodic group forms +1 ; Which group tends to not form ions or react? The group that tends to form 1+ ions is group 1,.
Electron Configurations of Ions Chemistry Steps
Which periodic group forms +1 ; The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Study with quizlet and memorize flashcards containing terms like which group tends to. The charge on a ion indicates the no. Which group tends to not form ions or react?
Do Metals Form Positive Or Negative Ions
The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The group that tends to.
Charge Of Ions Calculator
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which periodic group forms +1 ; The charge on a ion indicates the no. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$.
metals tend to form what kind of ions Lombardi Bothe1936
Which periodic group forms +1 ; Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Which group tends to not form ions or react? The charge on a ion indicates the no.
PPT 1 Name the ions formed by these elements and classify them as
The charge on a ion indicates the no. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The group that tends to form 1+.
The Elements In Group 1 Of The Periodic Table (Alkali Metals) Tend To Form +1 Ions.
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which periodic group forms +1 ; The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$.
Which Group Tends To Not Form Ions Or React?
The charge on a ion indicates the no. Study with quizlet and memorize flashcards containing terms like which group tends to.