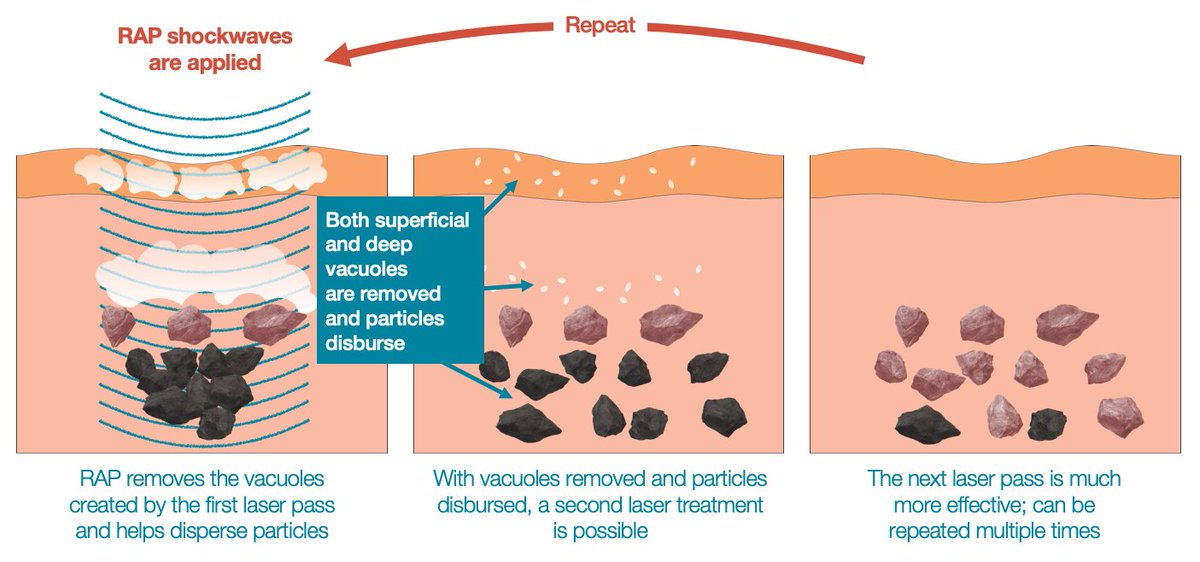
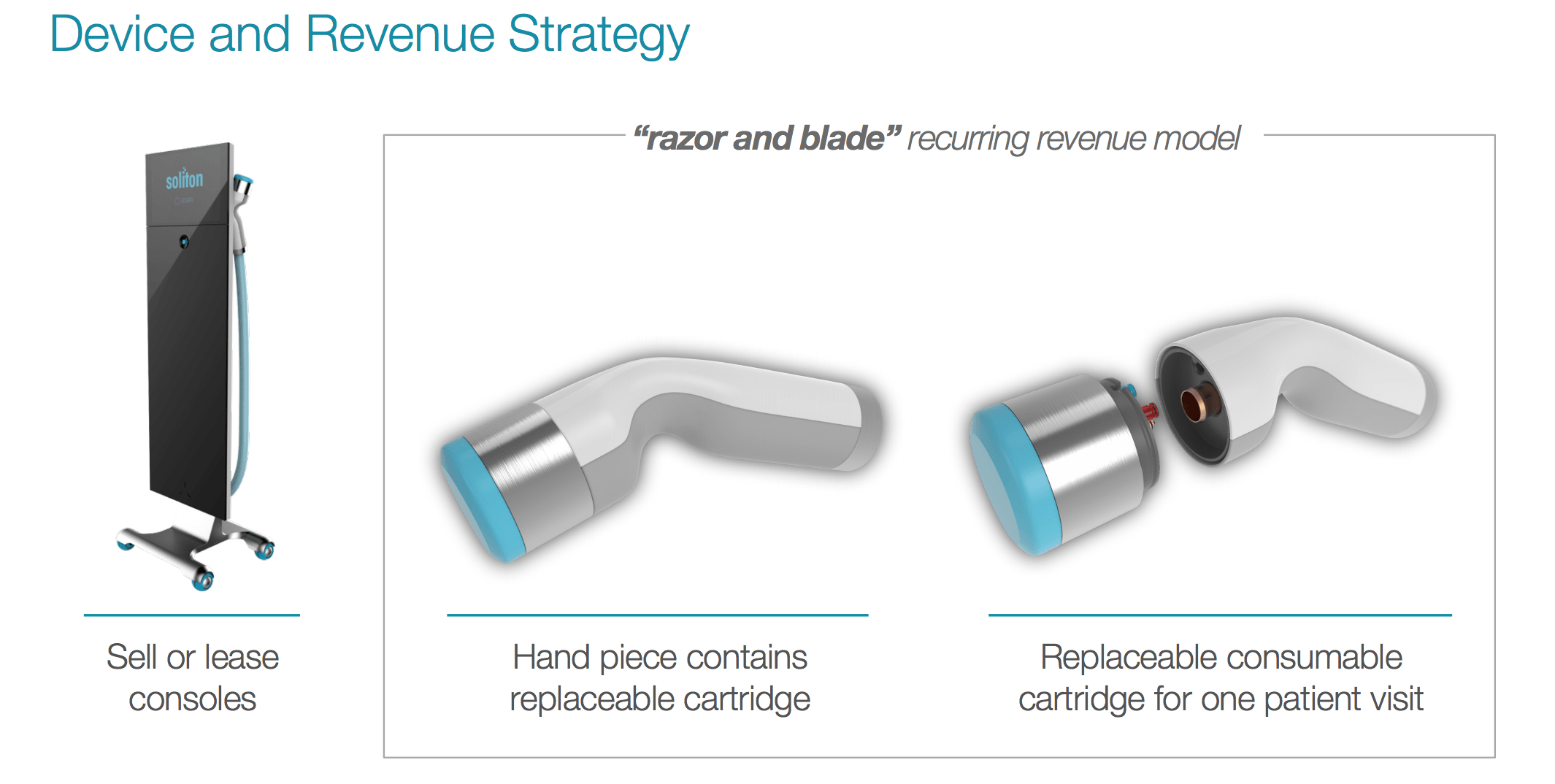
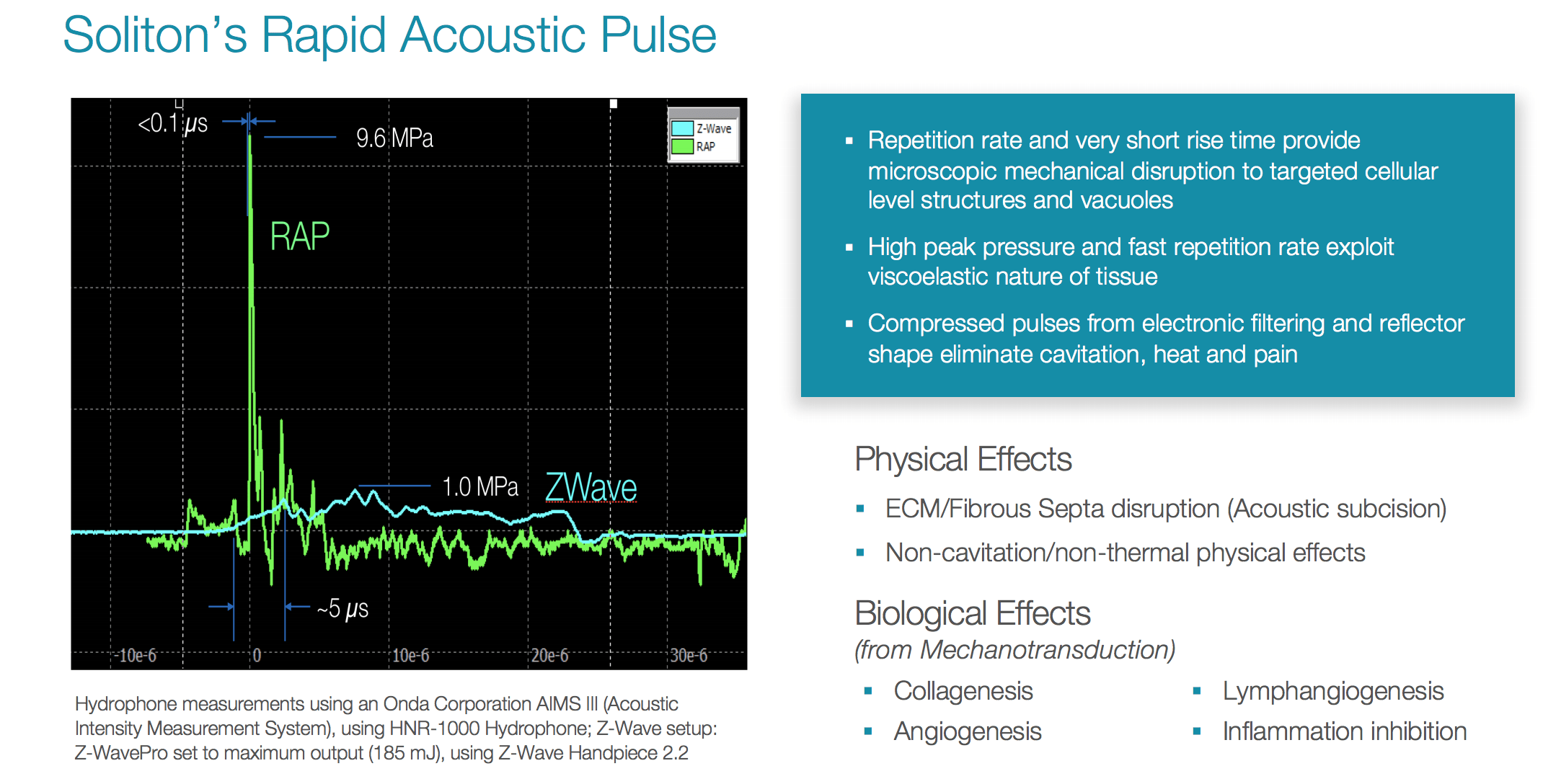
Soliton Tattoo Removal - 510(k) for modifications to its resonic device. Food and drug administration granted soliton, inc. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal.
Food and drug administration granted soliton, inc. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. 510(k) for modifications to its resonic device.
Food and drug administration granted soliton, inc. 510(k) for modifications to its resonic device. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal.
Soliton Tattoo Removal Uk MREBU
Food and drug administration granted soliton, inc. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. 510(k) for modifications to its resonic device.
Soliton Will Deliver RESONIC DualPlatform Device June 2021 Plastic
Food and drug administration granted soliton, inc. 510(k) for modifications to its resonic device. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal.
Acoustically Powered Tattoo Removers Soliton Rapid Acoustic Pulse System
Food and drug administration granted soliton, inc. 510(k) for modifications to its resonic device. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal.
Soliton Soars a Second Day After FDA Approves Tattoo Removal Device
510(k) for modifications to its resonic device. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. Food and drug administration granted soliton, inc.
Soliton Slow, Steady Progress Could Win Tattoo & Cellulite Removal
Food and drug administration granted soliton, inc. 510(k) for modifications to its resonic device. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal.
Soliton Acoustic Shockwave Device to Aid in Tattoo Removal Medgadget
Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. 510(k) for modifications to its resonic device. Food and drug administration granted soliton, inc.
Soliton Tattoo Removal Uk MREBU
Food and drug administration granted soliton, inc. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. 510(k) for modifications to its resonic device.
Soliton Slow, Steady Progress Could Win Tattoo & Cellulite Removal
Food and drug administration granted soliton, inc. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. 510(k) for modifications to its resonic device.
Soliton Faster Tattoo Removal Using RAP Technology Tattoo removal
510(k) for modifications to its resonic device. Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. Food and drug administration granted soliton, inc.
Food And Drug Administration Granted Soliton, Inc.
Soliton has completed several stages of clinical trials in testing our rapid acoustic pulse (rap) technology for tattoo removal. 510(k) for modifications to its resonic device.