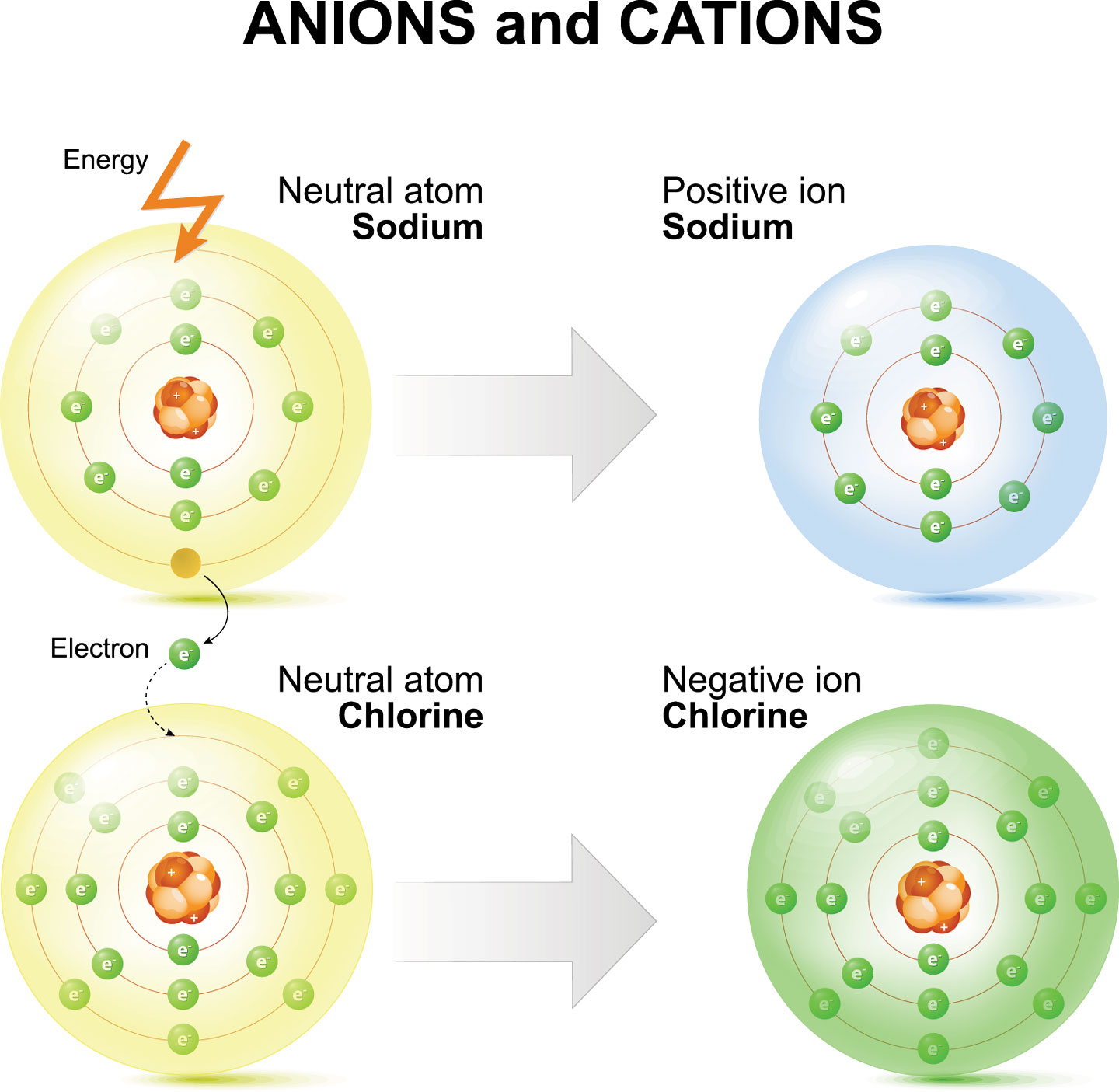
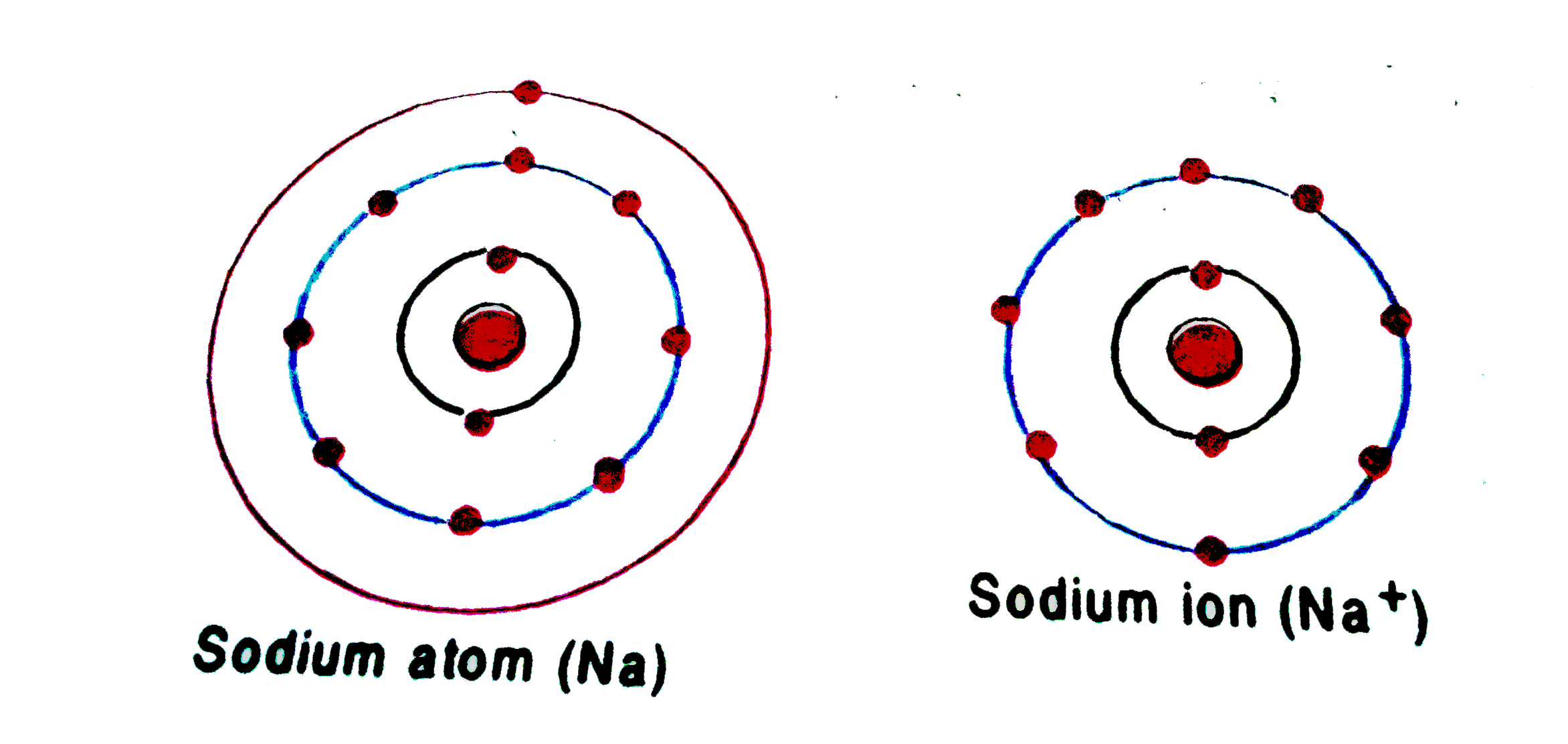
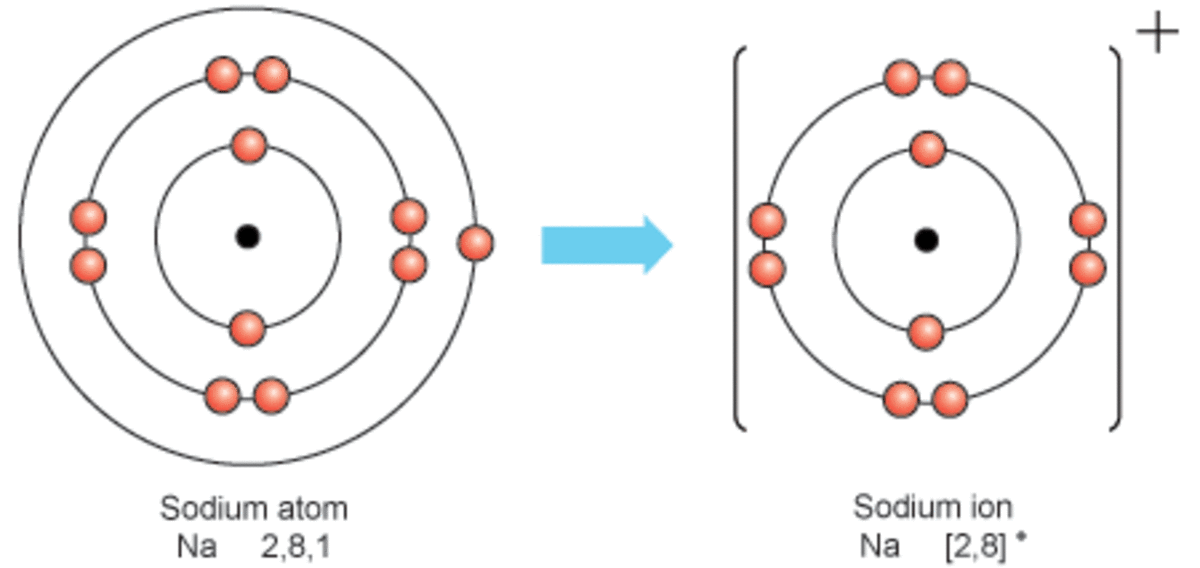
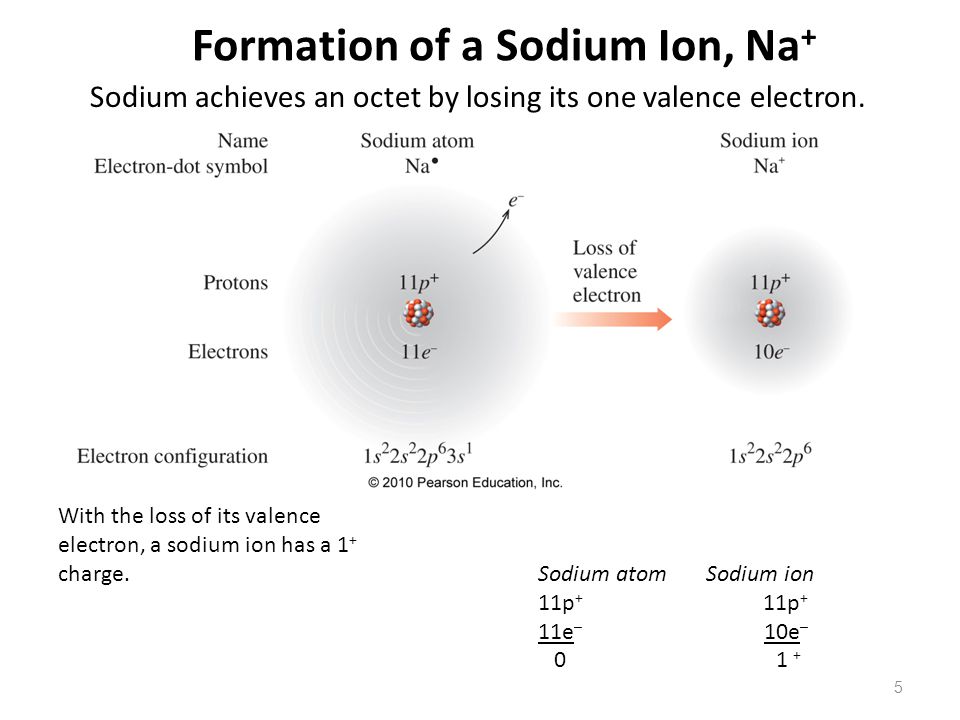
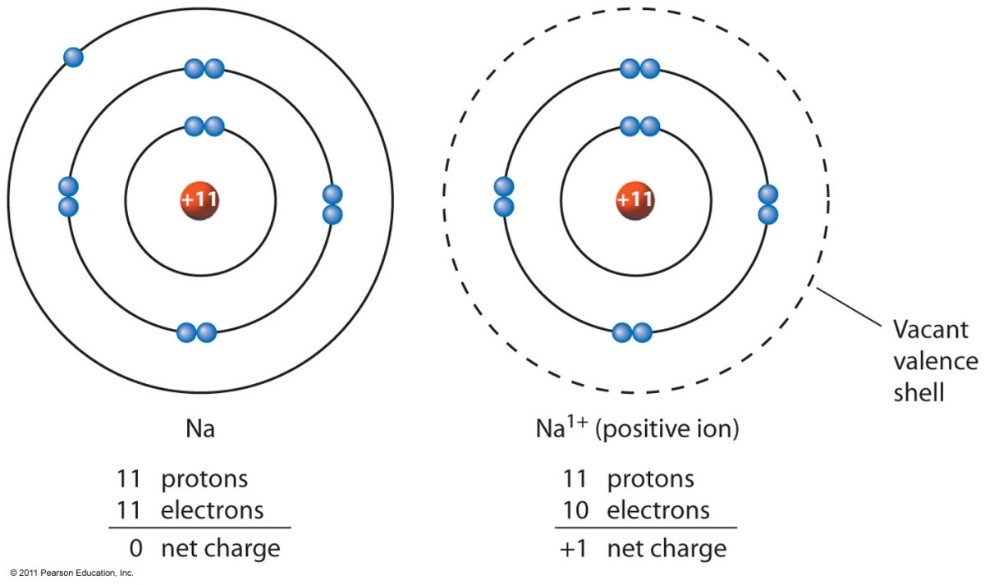
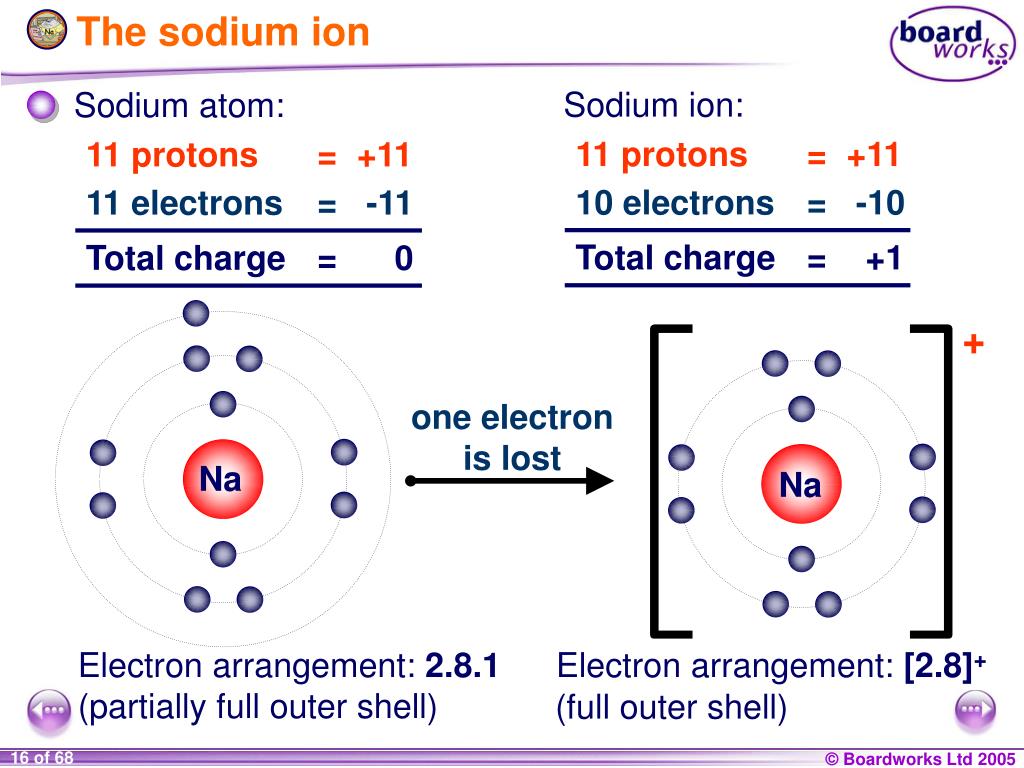
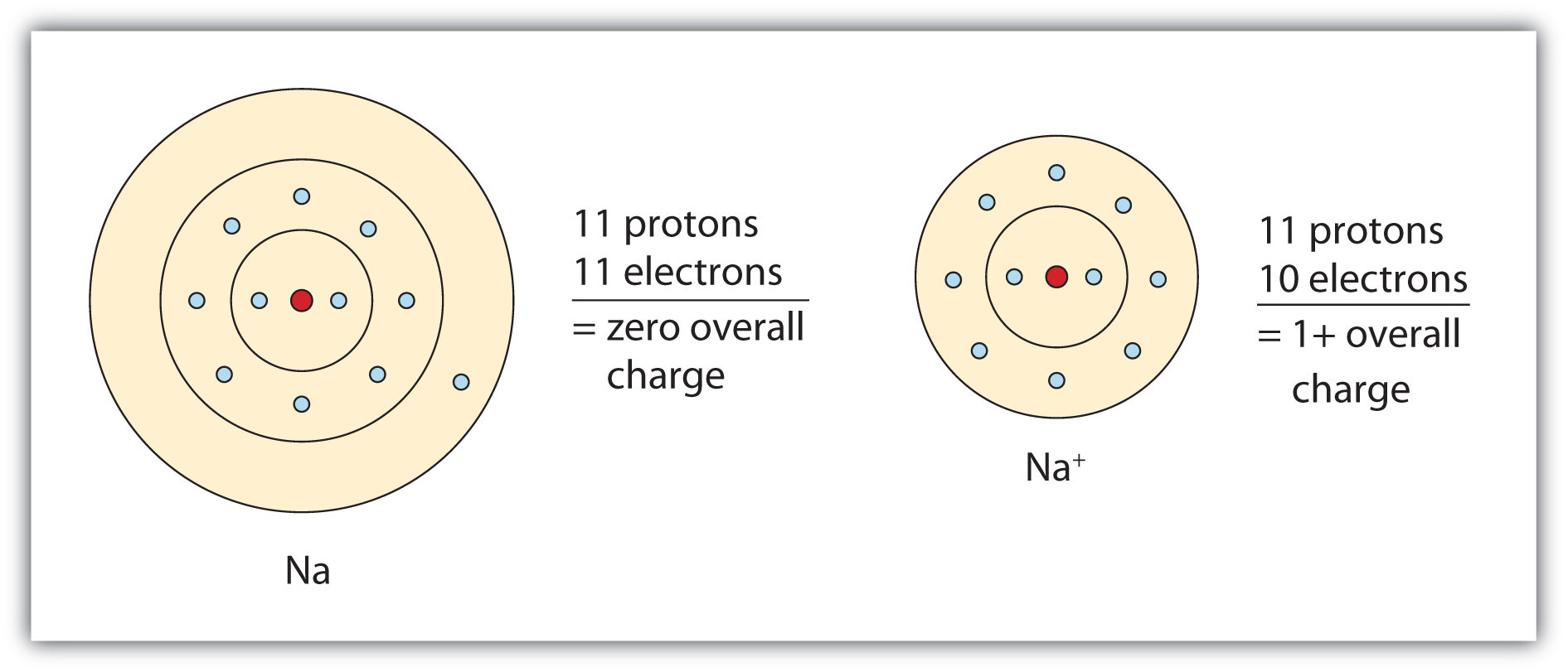
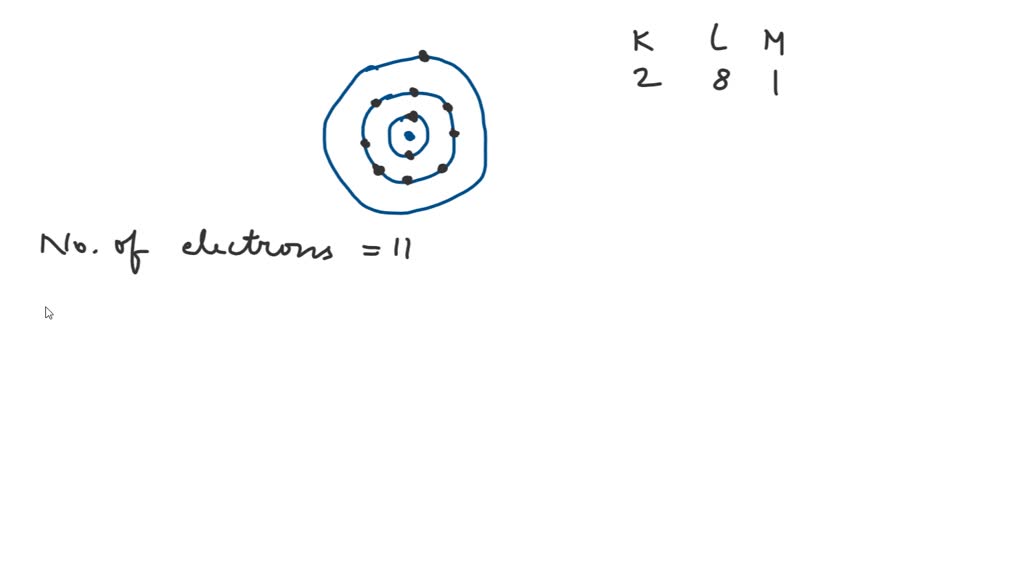
Sodium Forms An Ion With A Charge Of - Well, we form a na^+ ion. The sodium ion still has. The sodium atom loses its outer electron to become a sodium ion. Sodium metal is easily oxidized. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. For example, in the compound sodium chloride — table salt — the sodium.
For example, in the compound sodium chloride — table salt — the sodium. The sodium ion still has. The sodium atom loses its outer electron to become a sodium ion. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Well, we form a na^+ ion. Sodium metal is easily oxidized.
The sodium atom loses its outer electron to become a sodium ion. Well, we form a na^+ ion. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. For example, in the compound sodium chloride — table salt — the sodium. The sodium ion still has. Sodium metal is easily oxidized.
Explainer Ions and radicals in our world Science News for Students
The sodium ion still has. Well, we form a na^+ ion. The sodium atom loses its outer electron to become a sodium ion. Sodium metal is easily oxidized. For example, in the compound sodium chloride — table salt — the sodium.
Sodium Electron Configuration Electron Configuration Sodium What is
For example, in the compound sodium chloride — table salt — the sodium. Sodium metal is easily oxidized. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The sodium ion still has. Well, we form a na^+ ion.
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind
The sodium ion still has. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Sodium metal is easily oxidized. The sodium atom loses its outer electron to become a sodium ion. Well, we form a na^+ ion.
subatomic particles Montessori Muddle
The sodium ion still has. Sodium metal is easily oxidized. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Well, we form a na^+ ion. For example, in the compound sodium chloride — table salt — the sodium.
Sodium Electron Configuration (Na) with Orbital Diagram
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The sodium atom loses its outer electron to become a sodium ion. The sodium ion still has. Well, we form a na^+ ion. Sodium metal is easily oxidized.
Sodium Forms an Ion With a Charge of JasminehasGillespie
For example, in the compound sodium chloride — table salt — the sodium. The sodium atom loses its outer electron to become a sodium ion. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. The sodium ion still has. Well, we form a na^+ ion.
Ions Types, Summary, Classification & Facts
The sodium ion still has. The sodium atom loses its outer electron to become a sodium ion. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. For example, in the compound sodium chloride — table salt — the sodium. Sodium metal is easily oxidized.
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898
For example, in the compound sodium chloride — table salt — the sodium. Well, we form a na^+ ion. The sodium ion still has. Sodium metal is easily oxidized. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
Ions
Well, we form a na^+ ion. Sodium metal is easily oxidized. The sodium atom loses its outer electron to become a sodium ion. For example, in the compound sodium chloride — table salt — the sodium. The sodium ion still has.
When Sodium Atoms Form Ions, They Always Form A 1+ Charge, Never A 2+ Or 3+ Or Even 1− Charge.
Well, we form a na^+ ion. The sodium ion still has. For example, in the compound sodium chloride — table salt — the sodium. The sodium atom loses its outer electron to become a sodium ion.